

Each amino acid has the same fundamental structure, which consists of a central carbon atom, or the alpha ( α) carbon, bonded to an amino group (NH 2), a carboxyl group (COOH), and to a hydrogen atom. Amino AcidsĪmino acids are the monomers that comprise proteins. Two rare new amino acids were discovered recently (selenocystein and pirrolysine), and additional new discoveries may be added to the list. Different arrangements of the same 20 types of amino acids comprise all proteins. Changes in temperature, pH, and exposure to chemicals may lead to permanent changes in the protein's shape, leading to loss of function, or denaturation. Protein shape is critical to its function, and many different types of chemical bonds maintain this shape. For example, hemoglobin is a globular protein, but collagen, located in our skin, is a fibrous protein. Some proteins are globular in shape whereas, others are fibrous in nature. Proteins have different shapes and molecular weights. Table 3.1 lists the primary types and functions of proteins. For example, insulin is a protein hormone that helps regulate the blood glucose level. Hormones are chemical-signaling molecules, usually small proteins or steroids, secreted by endocrine cells that act to control or regulate specific physiological processes, including growth, development, metabolism, and reproduction. An example of an enzyme is salivary amylase, which hydrolyzes its substrate amylose, a component of starch. Note that all enzymes increase the reaction rate and, therefore, are organic catalysts. Those that build more complex molecules from their substrates are anabolic enzymes, and enzymes that affect the rate of reaction are catalytic enzymes.

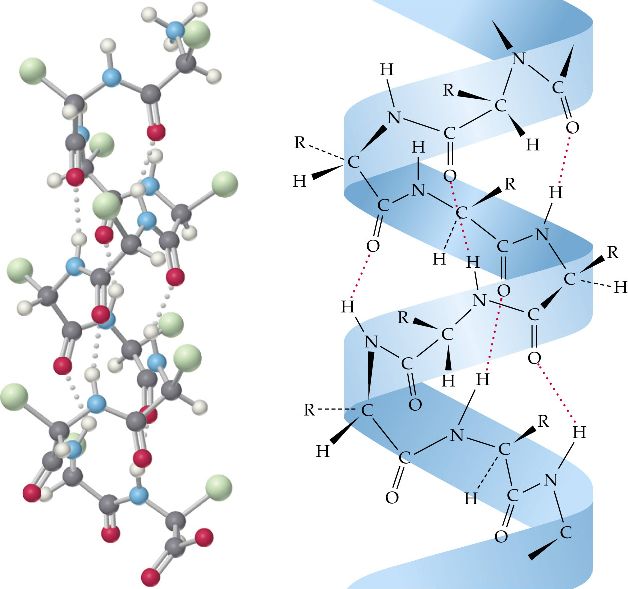
We call enzymes that break down their substrates catabolic enzymes. The enzyme may help in breakdown, rearrangement, or synthesis reactions. Each enzyme is specific for the substrate (a reactant that binds to an enzyme) upon which it acts. Types and Functions of ProteinsĮnzymes, which living cells produce, are catalysts in biochemical reactions (like digestion) and are usually complex or conjugated proteins. They are all, however, amino acid polymers arranged in a linear sequence. Their structures, like their functions, vary greatly. Each cell in a living system may contain thousands of proteins, each with a unique function. They may serve in transport, storage, or membranes or they may be toxins or enzymes. Proteins may be structural, regulatory, contractile, or protective. Proteins are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. Describe the ways in which protein shape and function are linked.Explain the four levels of protein organization.Discuss the relationship between amino acids and proteins.Describe the functions proteins perform in the cell and in tissues.

The characterizations and classifications made in this study prompt a reevaluation of constraints used in structure prediction efforts.By the end of this section, you will be able to do the following: This distribution is in accordance with structure types expected if the helix macro dipole effect makes a substantial contribution to the stability of the native structure. In 12 of these bundles, all pairs of neighboring helices were oriented in an anti-parallel fashion. Angular requirements further reduced the list of bundles to 13. Application of an analytical method that examines the difference between solvent-accessible surface areas in packed and partially unpacked bundles reduced the number of structures to 16. An exhaustive examination of the Brookhaven Crystallographic Protein Data Bank and other literature sources has lead to the discovery of 20 putative four-alpha-helix bundles. The four-alpha-helix bundle, a common structural motif in globular proteins, provides an excellent forum for the examination of predictive constraints for protein backbone topology.


 0 kommentar(er)
0 kommentar(er)
